Two infants with leukaemia are now in remission, thanks to a world-first treatment that uses genetically engineered T-cells from healthy donors.
The UK patients were first given the treatment back in 2015, after chemotherapy failed to show results. Now, after two years, both remain cancer-free.
If similar success is seen in future trials, the treatment could offer a cheap and universal way to fight cancers, without needing to tailor T-cells specifically for different patients.
"This application of an emerging technology has provided a demonstration of the potential of gene-editing strategies for engineered cell therapies, albeit with a clinical experience limited to two infants," the team from London's Great Ormond Street Hospital writes.
The infants, who were 11 months and 16 months when they started the new treatment, had previously undergone chemotherapy to treat leukaemia. Both treatments failed to show any results, and their parents were told they should prepare for the worst.
With no other options, doctors at London's Great Ormond Street Hospital tried a new procedure: injecting the infants with genetically engineered T-cells - known as chimeric antigen receptor, or CAR-T cells - taken from healthy donors.
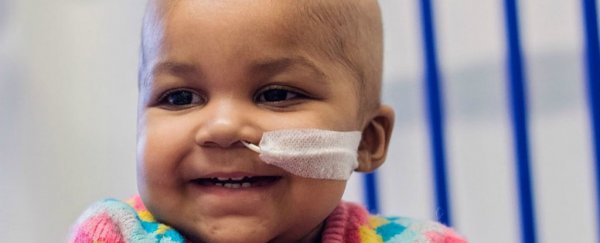
"I didn't want to go down that road, I'd rather that she tried something new and I took the gamble," Ashleigh Richards, father of one of the infants, Layla Richards (pictured above), told James Gallagher at the BBC shortly after the treatments in 2015.
"And this is her today standing laughing and giggling, she was so weak before this treatment, it was horrible and I'm just thankful for this opportunity."
T-cells are a type of white blood cell that can attack and destroy infected cells within the body, including cancer cells.
Unfortunately, the body's T-cells aren't always up to the task of finding and destroying all cancerous cells, especially if they're growing particularly fast.
There have been attempts in the past to improve T-cells' ability specifically seek and destroy cancer cells.
One of the most successful ways involves doctors taking a patient's own T-cells, genetically engineering them to better target errant cells, and placing them back in the body, where they reproduce and form a more aggressive army.
But not all patients have enough heathy cells for this to work.
This new treatment, on the other hand, doesn't require a patient's own T-cells, meaning doctors could soon have genetically engineered treatments ready to be implemented as soon as cancer is diagnosed, instead of having to wait for the patient's own T-cells to be engineered.
"We're in a wonderful place compared to where we were five months ago, but that doesn't mean cure," team member Paul Veys from University College London told the BBC back in 2015.
"The only way we will find out if this is a cure is by waiting that one or two years, but even having got this far from where we were is a major, major step."
It has now been one to two years, and both children are still in remission, suggesting that the treatment has worked so far.
It's important to note that the treatment has only been performed on two patients, so there will need to be more successful trials involving a much larger group of patients before the treatment is widely available for other patients.
But it's exciting to see the results so far, particularly when it means saving the lives of two babies.
Besides the treatment's apparent success, having an off-the-shelf treatment would be a whole lot cheaper than genetically engineering each patient's own T-cells.
This new process would involve using the blood of donors to create large batches of CAR-T that could be frozen and given out in doses.
"We estimate the cost to manufacture a dose would be about US$4,000," CAR-T developer Julianne Smith, from a biopharmaceutical company called Cellectis that develops immunotherapies for cancer, told MIT Technology Review.
Smith, who was not involved in the research, says using a patient's own T-cells costs roughly US$50,000 a pop, making the new treatment far more affordable.
Hopefully, as the research continues, more doctors will test the team's treatment. If successful, the treatment could see use in hospitals all around the world, but only time will tell.
The team's work has been published in Science Translational Medicine.